Student Research Symposium Program Portal: Submission #104
Submission information
Submission Number: 104
Submission ID: 8086
Submission UUID: 9e0f3ac0-f89e-4beb-bdb0-626e8f578b21
Submission URI: /student-research/symposium/research-symposium-program-portal
Submission Update: /student-research/symposium/research-symposium-program-portal?token=_34bv87hkRIrcSB5gJ-bNN_8FTixM_6PowD_UkoLyio
Created: Fri, 02/07/2025 - 03:48 PM
Completed: Fri, 02/07/2025 - 03:52 PM
Changed: Mon, 04/14/2025 - 12:50 PM
Remote IP address: 73.118.9.19
Submitted by: Anonymous
Language: English
Is draft: No
Webform: Research Symposium Program Portal WF
Submitted to: Student Research Symposium Program Portal
Wajeeha
Raqeeb
{Empty}

Psychology
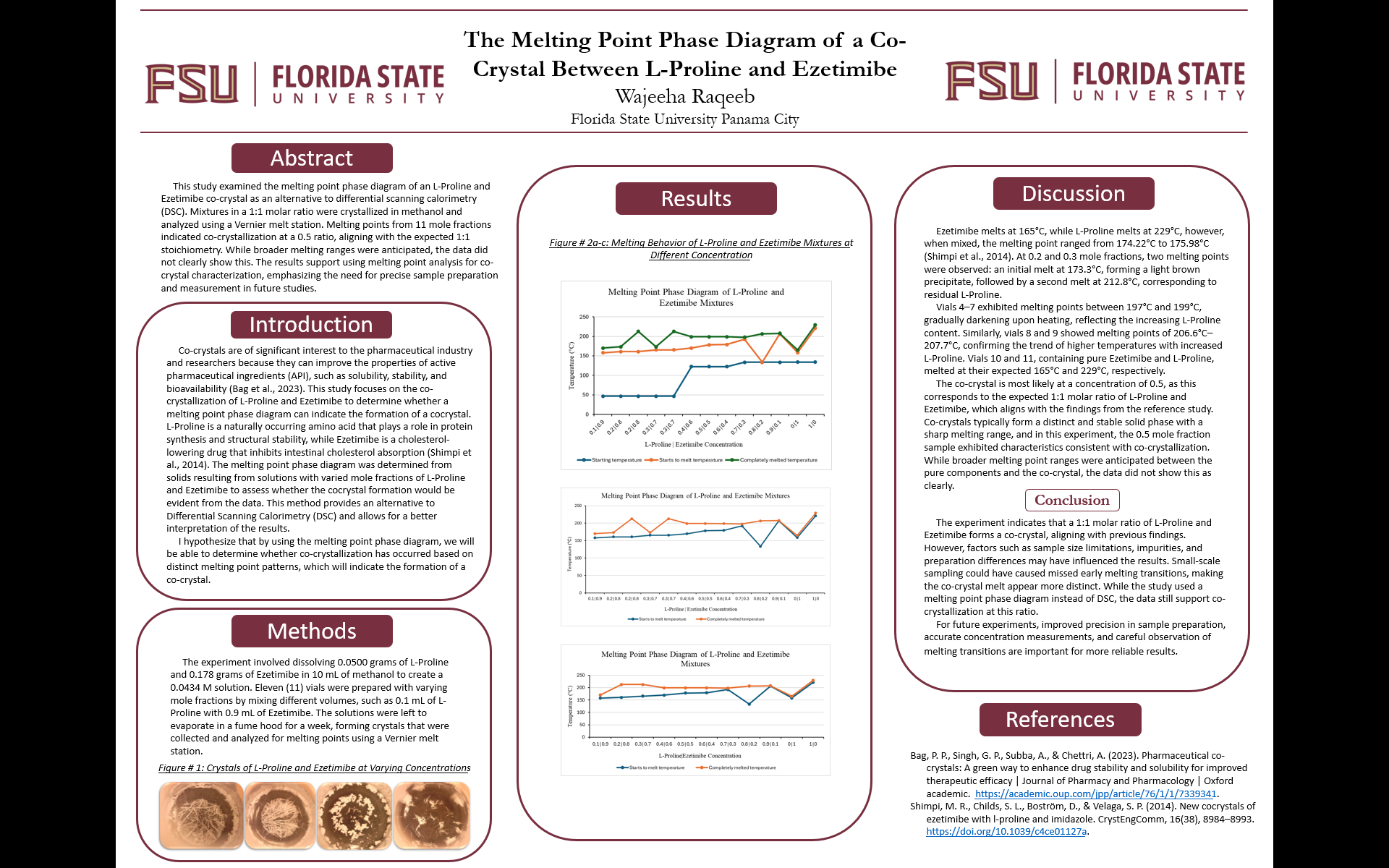
I am Wajeeha from Pakistan, a senior majoring in psychology with a pre-med track. My research focuses on the thermal properties of cocrystals, specifically the melting point phase diagram of an L-Proline and Ezetimibe cocrystal. Beyond research, my long-term goal is to attend medical school and become a psychiatrist to better understand and support people in managing their mental health.
The melting point phase diagram of a cocrystal between L-Proline and Ezetimibe
Cocrystals are of significant interest to the pharmaceutical industry and researchers because they can improve the properties of active pharmaceutical ingredients (API), such as solubility, stability, and bioavailability. In the article "New cocrystals of ezetimibe with L-proline and imidazole" by Shimpi et al., Differential Scanning Calorimetry (DSC) apparatus was used to investigate the thermal properties of this cocrystal. L-Proline is a naturally occurring amino acid that plays a role in protein synthesis and structural stability, while Ezetimibe is a cholesterol-lowering drug that inhibits intestinal cholesterol absorption. The cocrystal of L-Proline and Ezetimibe is being examined for its thermal properties, specifically through the melting point phase diagram. However, the melting point phase diagram was determined from the solids resulting from solutions with varied mole fractions of L-Proline and Ezetimibe to see if the cocrystal would be evident from the data.
Therefore, the purpose of the current research is to determine and present the melting point phase diagram of this cocrystal. This method provides an alternative to DSC and allows for a better understanding of its thermal behavior.
Therefore, the purpose of the current research is to determine and present the melting point phase diagram of this cocrystal. This method provides an alternative to DSC and allows for a better understanding of its thermal behavior.
Paul Baures
Florida State University-Panama City
Chemistry
pbaures@pc.fsu.edu
{Empty}
{Empty}
Cocrystal, L-Proline, Ezetimibe, melting point and phase diagram
C - 5 R - 5
Complete
Face to Face Poster session
Ochm Research Poster.pdf583.88 KB
No
2025
5th annual Undergraduate Research Symposium, April 17, 2025
https://pc.fsu.edu/student-research/symposium/research-symposium-program-portal?element_parents=elements/student_photo&ajax_form=1&_wrapper_format=drupal_ajax&token=_34bv87hkRIrcSB5gJ-bNN_8FTixM_6PowD_UkoLyio
{Empty}
